Since their description in the 1970s1, mesenchymal stem cells (MSC) have been at the center of very active research with the challenge of using them as therapy in organ and tissue repair. For that purpose, it has been essential to develop culture protocols that would ensure their growth and, eventually, their differentiation into specific tissues2–8.
This living sample of human MSC from bone marrow kindly provided by PromoCell GmbH, was pre-coated with human fibronectin and cultured with low-serum cell growth medium.
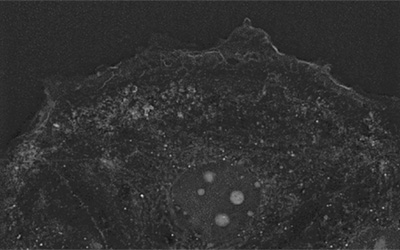
Once incubated, the sample was observed under the 3D Cell Explorer. Nanolive imaging is a method of choice for stem cell imaging, as its technology avoids intense light exposure or labelling-induced perturbations, which limit the usage of imaging methods like fluorescence microscopy.
Human mesenchymal stem cells growing on fibronectin
This blogpost and our previous mitosis in human mesenchymal stem cells (MSCs) blog post feature the same cell type. However, the MSCs presented here grow on fibronectin, which resulted in a difference in shape (Figure 1). Fibronectin is a glycoprotein from the extracellular matrix that enhances attachment and spreading of cells cultured in vivo and in vitro9.
The use of fibronectin as substrate influences the cytoskeletal structures of the growing cells, leading to the formation of stress fibers in this case.
Stress fibers appear in most cell types, but most commonly on those of mesenchymal origin10 (Figure 2). Actin can be found in the cytosol as a monomer (G-actin) or as a polymer (F-actin). Stress fibers are bundles of highly compacted filamentous actin resulting from actin polymerization, that are associated with several cytoskeletal proteins like myosin II and α-actinin. They are connected to the underlying matrix -fibronectin in our case-, at one or both ends in structures known as focal adhesions1.
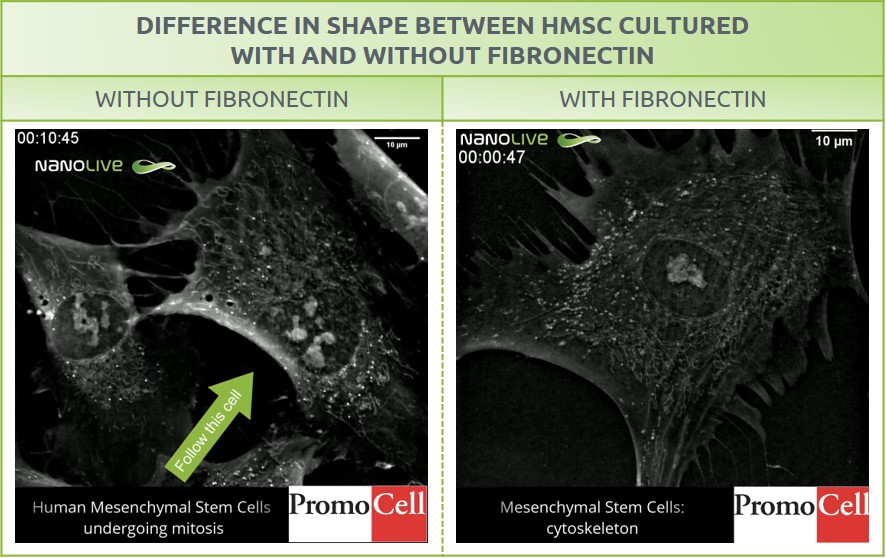
Figure 1: Difference in shape between human mesenchymal stem cells cultured without (left) and with (right) fibronectin.
Similarly to the distinguishable metaphase plate on our mitosis blog post, the high concentration of proteins in stress fibers leads to a detectable refractive index that allows us to visualize the structure under the microscope (Figure 2).
Stress fibers play a role in stem cell differentiation into specific tissues11.
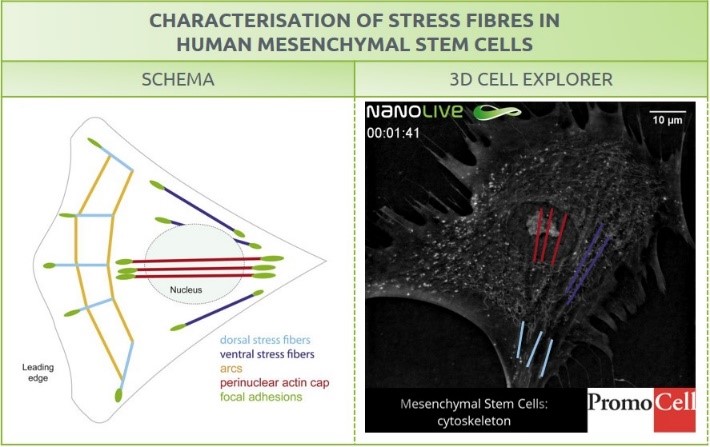
Figure 2. Characterization of stress fibers in human mesenchymal stem cells. Right: schema from Burridge, K., & Guilluy, C. (2016). Focal adhesions, stress fibers and mechanical tension. Experimental cell research, 343(1), 14–20. Right: Image of hMSC obtained with the 3D Cell Explorer. Stress fibers are highlighted
- Dexter, T. M., Allen, T. D. & Lajtha, L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J. Cell. Physiol. 91, 335–344 (1977).
- Wolfe, M., Pochampally, R., Swaney, W. & Reger, R. L. Isolation and Culture of Bone Marrow-Derived Human Multipotent Stromal Cells (hMSCs). in Mesenchymal Stem Cells 3–25 (Humana Press, 2008). doi:10.1007/978-1-60327-169-1_1
- Pittenger, M. F. Mesenchymal Stem Cells from Adult Bone Marrow. in Mesenchymal Stem Cells 27–44 (Humana Press, 2008). doi:10.1007/978-1-60327-169-1_2
- Reger, R. L., Tucker, A. H. & Wolfe, M. R. Differentiation and Characterization of Human MSCs. in Mesenchymal Stem Cells 93–107 (Humana Press, 2008). doi:10.1007/978-1-60327-169-1_7
- Zigova, T., Sanberg, P. R. & Sanchez-Ramos, J. R. Neural stem cells : methods and protocols. (Humana Press, 2002).
- Fraser, J. K., Zhu, M., Wulur, I. & Alfonso, Z. Adipose-Derived Stem Cells. in Mesenchymal Stem Cells 59–67 (Humana Press, 2008). doi:10.1007/978-1-60327-169-1_4
- Mesenchymal Stem Cells. (Humana Press, 2008). doi:10.1007/978-1-60327-169-1
- Mesenchymal Stem Cell Culture | PromoCell. Available at: https://www.promocell.com/portfolio/mesenchymal-stem-cell-culture/#application-notes. (Accessed: 20th May 2019)
- Birdwell, C. R., Gospodarowicz, D. & Nicolson, G. L. Identification, localization, and role of fibronectin in cultured bovine endothelial cells. Proc. Natl. Acad. Sci. 75, 3273–3277 (1978).
- Burridge, K. & Guilluy, C. Focal adhesions, stress fibers and mechanical tension. Exp. Cell Res. 343, 14–20 (2016).
- Steward, A. J. & Kelly, D. J. Mechanical regulation of mesenchymal stem cell differentiation. J. Anat. 227, 717–31 (2015).
Read our latest news
Revolutionizing lipid droplet analysis: insights from Nanolive’s Smart Lipid Droplet Assay Application Note
Introducing the Smart Lipid Droplet Assay: A breakthrough in label-free lipid droplet analysis Discover the power of Nanolive's Smart Lipid Droplet Assay (SLDA), the first smart digital assay to provide a push-button solution for analyzing lipid droplet dynamics,...
Food additives and gut health: new research from the University of Sydney
The team of Professor Wojciech Chrzanowski in the Sydney Pharmacy School at the University of Sydney have published their findings on the toxic effect of titanium nanoparticles found in food. The paper “Impact of nano-titanium dioxide extracted from food products on...
2023 scientific publications roundup
2023 has been a record year for clients using the Nanolive system in their scientific publications. The number of peer-reviewed publications has continued to increase, and there has been a real growth in groups publishing pre-prints to give a preview of their work....
Nanolive microscopes

3D CELL EXPLORER
Budget-friendly, easy-to-use, compact solution for high quality non-invasive 4D live cell imaging

3D CELL EXPLORER-fluo
Multimodal Complete Solution: combine high quality non-invasive 4D live cell imaging with fluorescence

CX-A
Automated live cell imaging: a unique walk-away solution for long-term live cell imaging of single cells and cell populations