Call to all microscopists!
Nanolive has had a number of scientific discussions recently on whether fluorescence based live cell imaging observations on cell/organelle dynamics and morphology can lead to incorrect conclusions if phototoxicity is not controlled for.
Visit our dedicated page on phototoxicity here: https://www.nanolive.ch/technology/overcoming-phototoxicity/.
As such we are proposing an open challenge to all live cell researchers using fluorescence based microscopy techniques.
Here is the null hypothesis: fluorescence based imaging can successfully be used to image living cells for over 48 hours with zero observable negative impact.
To prove this, email us with subject: “The fluorescence challenge” and any fluorescence imaging video of live cells lasting longer than 48 hours that clearly show healthy mitochondria (the organelle most likely to be observably impacted by fluorescence based imaging) beyond 48 hours.
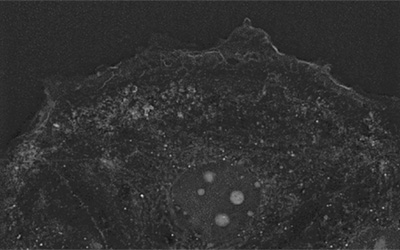
Featured video: Preadipocyte cell.
Dapi exposition led to complete cell shrinkage and rounding, followed by extensive blebbing at all intensities with 100ms exposure time. At 10% intensity and 100ms exposure time, loss of cell adhesion and cell shrinkage took place in 8 minutes.
Imaging regimen: 30 minutes acquisition at a frequency of 1 image every 6 seconds.
To be eligible for the prize in addition to the time-lapse/movie we need:
- Name of microscope used
- Cost of Microscope
- Cost of fluorescence label
- Cell Type and experimental conditions (including frequency of imaging)
Prize
For any lab able to send us imaging over 48 hours long, showing mitochondria labelled via fluorescence and appearing healthy, we will send you a bottle of champagne and share your movie on social media to celebrate your success.
If no one is able to pass 48 hours we will send a bottle of champagne to the lab that provides the longest fluorescence based imaging of mitochondria before they show signs of damage.
As a comparison here is a label-free movie by Nanolive
Name of microscope: 3D Cell Explorer
Cost of Microscope: microscopy <30K Euro, incubator <10k Euro
Cost of fluorescence label: 0
Cell Type and experimental conditions (including frequency of imaging): Live imaging of mouse pre-adipocytes for 48 hours. 1 image per minute.
Special thanks to the following entries:
Name of microscope: 3D Cell Explorer-fluo
Cost of Microscope: microscopy <50K Euro
Cell Type and experimental conditions (including frequency of imaging): WM793 melanoma cells stained with Hoechst and Mitotracker Deep red. Cells were imaged every 30sec for 6h and excited with DAPI and Cy5 filter every 5 min during 6h. This video was kindly provided by the Cancer Research Center in Lyon. With special thanks to Gabriel Ichim, director of the Cancer Cell Death research team.
Experiment description:
Microscope setup
Nikon Ti2 with Okolabs incubator and PFS (Perfect Focus System) between 50’000 and 100’000 €
CFI Plan Fluor 40x oil 1.3 objective:around 10’000€
ReScan Confocal add-on (RCM) from Confocal.nl
Omicron 561nm 20mW laser
Hamamatsu Orca Flash 4.0 V3 camera
Acquisition details:
Frame rate: 1 frame per 10 seconds
Duration: 61h06m
2048*2044 pixels
Laserpower: 1 microwatt at sample plane
Pixel dwell time: about 1 microsecond
Sample details:
Cell type: HO1N1 cells
Staining: Bacmam 2.0 Mitochrondria RFP (Invitrogen™ C10601)
Glass bottom 8-well IBIDI chamber
Prolong Live 1:100 (LIFE TECHNOLOGIES P36975)
Cost of microscope:
Nikon Ti2 with Okolabs incubator and PFS (Perfect Focus System): unknow
CFI Plan Fluor 40x oil 1.3 objective: unknown
ReScan Confocal add-on: <40k EUR
Cost of label: 10 EUR
Read our latest news
Revolutionizing lipid droplet analysis: insights from Nanolive’s Smart Lipid Droplet Assay Application Note
Introducing the Smart Lipid Droplet Assay: A breakthrough in label-free lipid droplet analysis Discover the power of Nanolive's Smart Lipid Droplet Assay (SLDA), the first smart digital assay to provide a push-button solution for analyzing lipid droplet dynamics,...
Food additives and gut health: new research from the University of Sydney
The team of Professor Wojciech Chrzanowski in the Sydney Pharmacy School at the University of Sydney have published their findings on the toxic effect of titanium nanoparticles found in food. The paper “Impact of nano-titanium dioxide extracted from food products on...
2023 scientific publications roundup
2023 has been a record year for clients using the Nanolive system in their scientific publications. The number of peer-reviewed publications has continued to increase, and there has been a real growth in groups publishing pre-prints to give a preview of their work....
Nanolive microscopes

3D CELL EXPLORER
Budget-friendly, easy-to-use, compact solution for high quality non-invasive 4D live cell imaging

3D CELL EXPLORER-fluo
Multimodal Complete Solution: combine high quality non-invasive 4D live cell imaging with fluorescence

CX-A
Automated live cell imaging: a unique walk-away solution for long-term live cell imaging of single cells and cell populations