Welcome to the June edition of the AI for Live Cell Insights Newsletter, bringing you the latest live cell analyses powering drug discovery and cosmetics development. Each month, we will explore a new application of AI-based cellular analysis for label-free live cell imaging, with publication highlights and news from Nanolive. This month, we are highlighting a new publication using deep learning to study organelle dynamics during coronavirus infection, and sharing our upcoming events.
Using deep learning to understand coronavirus infection at the subcellular level

Recently published in Nature Communications, the latest research from Nanolive’s Deep Quantitative Biology department and the Institut Pasteur uses cutting-edge AI-based computer vision to give new insights into organelle network disruption during coronavirus infection.
Building on the expertise used to develop Nanolive’s existing digital assays for cellular image analysis, members of Nanolive’s Deep Quantitative Biology team created new automated methods for detecting and quantifying cell nuclei, nucleoli and mitochondria in label-free holotomographic images. These digital segmentations of organelles were then used to quantify organelle numbers, their changing morphology, interactions, mass, and other features.
The granularity of this approach even made it possible to distinguish between the cellular responses to different coronavirus strains, which provoked different interaction patterns between organelles.
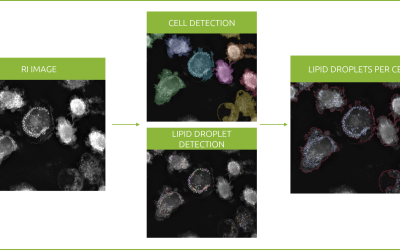
AI-powered segmentation of nuclei (cyan), nucleoli (pink), mitochondria (red), lipid droplets (green), and cell membrane (gray), shown next to the raw refractive index imaging data of U2OS cells (right).
Director of AI and Biology at Nanolive, Mathieu Fréchin, shared his enthusiasm for the opportunity to collaborate with the Institut Pasteur:
“Working with Nell, Olivier, Tim and all the other members of Pasteur was the greatest chance for the Deep Quantitative Department to develop an original quantitative biology approach to understand SARS-CoV-2 infection. We created bridges that we hope will nurture novel AI-fueled research.”
Read the open access full paper here.
Events
Nanolive’s Online Demo: Transforming drug discovery with AI-powered live cell analysis

Watch this exclusive demonstration tailored for biopharma professionals and academic researchers, showcasing Nanolive’s cutting-edge live cell imaging and analysis technology. Discover our unique holotomographic microscope and label-free imaging, enhanced by AI-powered software for effortless, push-button analysis, and learn how Nanolive’s technology can empower in vitro preclinical drug development, improve understanding of drug toxicity, and provide unbiased cellular insights.
Additionally, get a glimpse into the future of cell imaging analysis as Mathieu Fréchin discusses the team’s recent publication, highlighting the transformative potential of AI in advancing biopharmaceutical research and drug discovery.
Watch it here.
High-content Label-free Imaging: Spotlight on Applications for Live Phenotypic Analysis
20th August

In this webinar hosted by the American Society for Cell Biology (ASCB), discover the unique data gained from studying cell and organelle dynamics live and label-free. Holotomography is an innovative imaging technique which combines a rotating light source and the intrinsic refractive properties of cellular structures to produce crystal-clear, high resolution (200 nm) images of cells and their organelles, without labels or damage. Nanolive will showcase how such rich image data can be captured and processed automatically with AI-powered analysis to study cell death, T cell response, metabolism, and phenotypic responses. Then, we will take a deep-dive into case studies for a wide range of cell types and processes, from T cell killing, to mitochondrial networks, and phagocytosis. Come and see cells as you’ve never seen them before!
This event is open to members and non-members of ASCB.
Sign up here.
Latest publication highlights with Nanolive imaging:
- Drug delivery: Weglewska, E. et al. (2024) ‘Superior Drug Delivery Performance of Multifunctional Bilosomes: Innovative Strategy to Kill Skin Cancer Cells for Nanomedicine Application’, International Journal of Nanomedicine, https://doi.org/10.2147/IJN.S450181
- Mitochondria: Hong, W. et al. (2024) ‘PGC-1α loss promotes mitochondrial protein lactylation in acetaminophen-induced liver injury via the LDHB-lactate axis’, Pharmacological Research, https://doi.org/10.1016/j.phrs.2024.107228
- Oncology: Szlasa, W. et al. (2024) ‘Pulsed electric field induces exocytosis and overexpression of MAGE antigens in melanoma’, Scientific Reports, https://doi.org/10.1038/s41598-024-63181-x
- Alzheimers: Yang, I-H. et al. (2024) ‘Macrophage-mediated controlled release of cysteine protease inhibitor from PLGA-PEG/hydroxyapatite microspheres for targeting cathepsin S in Alzheimer’s disease’, European Polymer Journal, https://doi.org/10.1016/j.eurpolymj.2024.113151
- Oncology: Huang, K-H. et al. (2024) ‘GPR56/ADGRG1 induces biased Rho-ROCK-MLC and JAK-STAT3 signaling to promote amoeboid-like morphology and IL-6 upregulation in melanoma cells’, Research Square, https://doi.org/10.21203/rs.3.rs-4423390/v1

Morphological analysis of melanoma cells. Huang K-Y. et al. (2024)
- Immunology: Al Otaibi, A., et al. (2024) ‘Thapsigargin and Tunicamycin Block SARS-CoV-2 Entry into Host Cells via Differential Modulation of Unfolded Protein Response (UPR), AKT Signaling, and Apoptosis’, Cells, https://doi.org/10.3390/cells13090769
- Nanomedicine: Kurtuldu, F., et al. (2024) ‘Gallium-containing mesoporous nanoparticles influence in-vitro osteogenic and osteoclastic activity’, Biomaterials Advances, https://doi.org/10.1016/j.bioadv.2024.213922
- Nanomaterials: Ikeda, K. N., et al. (2024) ‘Dynamic microvilli sculpt bristles at nanometric scale’, Scientific Reports, https://doi.org/10.1038/s41467-024-48044-3
Find over 250 publications featuring Nanolive imaging here.
Subscribe to this monthly newsletter to stay up to date with AI applications in cell biology.
Read our latest news
Online Demo: Transforming drug discovery with AI-powered live cell analysis
Reveal cellular stories with AI Join us for an exclusive demonstration tailored for biopharma professionals and academic researchers, showcasing Nanolive’s cutting-edge live cell imaging and analysis technology. Discover our unique holotomographic microscope and...
Newsletter May 2024: In vitro drug candidate profiling
Welcome to the first edition of the AI for Live Cell Insights newsletter, bringing you the latest live cell analyses powering drug discovery and cosmetics development. Each month, we will explore a new application of AI-based cellular analysis for label-free live cell...
Revolutionizing lipid droplet analysis: insights from Nanolive’s Smart Lipid Droplet Assay Application Note
Introducing the Smart Lipid Droplet Assay: A breakthrough in label-free lipid droplet analysis Discover the power of Nanolive's Smart Lipid Droplet Assay (SLDA), the first smart digital assay to provide a push-button solution for analyzing lipid droplet dynamics,...